Resting Membrane Potential
可兴奋的细胞不产生冲动时,称为静息状态;处于静息状态的神经元膜内相对于膜外是负电荷,跨膜电荷存在着差异称为resting membrane potential
- Excitable Membrane: 可以产生动作电位的细胞的膜(神经元或者肌肉细胞)
The Chemical Basis
- cytosal and extracellular fluid: Water is the main solvent. Ions (Cations + and Anions −) are the charge carriers.
- phospholipid membrane: 提供屏障作用,由于磷脂双分子层是非极性的,导致离子无法通过
- Ion pumps: 细胞膜上的跨膜蛋白,可以允许特定离子通过细胞膜
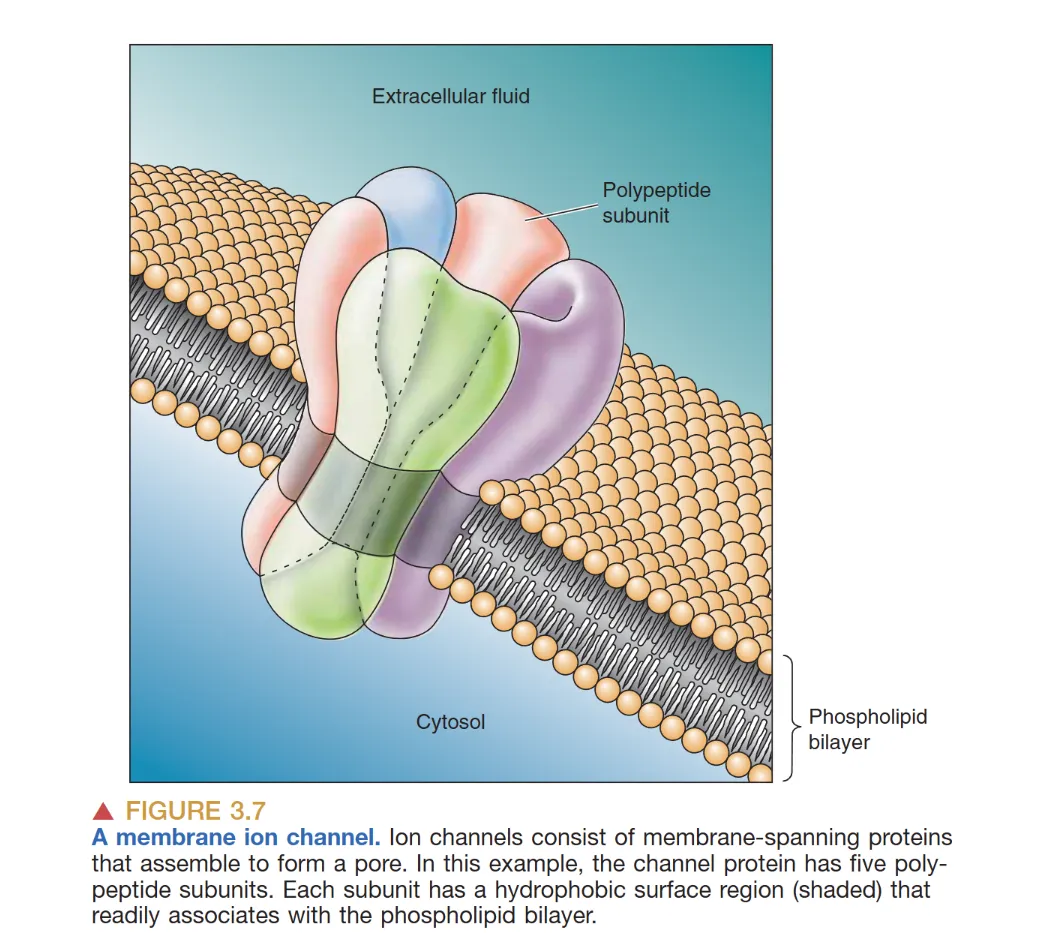
Movement of Ions
- 扩散: 通过离子通道顺浓度梯度扩散。
- 电场驱动: Movement of ions due to an electrical field (Current I, Voltage V, Conductance g).
Ion Basis of RMP
- 离子平衡电位(equilibrium potential):电场驱动力与浓度驱动力平衡,某种离子浓度梯度平衡
- 膜电位巨大改变由离子极小变化引起
- 净电荷差分布在膜内侧和外侧表面
- 离子驱动速度正比于driving force
- 已知某一离子浓度差,可计算离子平衡电位
Nernst Equation
Eion=2.303zFRTlog[ion]i[ion]o
这里E为某个离子的静息膜电位。
K+: in>out
Na+ Ca2+ Cl-: out>in
[!note] 37°C 时的简化公式
Eion=z61.65log[ion]i[ion]o
| 离子 | 浓度比 (外:内) | 平衡电位 (Eion) |
|---|
| K+ | 1 : 20 | -80 mV |
| Na+ | 10 : 1 | +62 mV |
| Ca2+ | 10,000 : 1 | +123 mV |
| Cl− | 11.5 : 1 | -65 mV |
GHK Equation
Nernst方程只考虑了单个离子如何流动,但细胞膜的离子是复杂的,所以GHK方程在Nernst方程基础上进行了拓展。
GHK Equation
The GHK equation is a fundamental fomula in neuroscience, extending the Nernst equation which is limited to a single ion species. It considers the relative permeabilities and concentrations of multiple ions.
Assumptions
Because the environment in the cell is very complex, we must build some assumptions to make the deriving process easy.
- Ionix Flux: the movement of ions across the membrane decribed by Nernst-Planck Equation which accounts for both concentration and electric potential gradients.
- The Electric Field is constant, letting us integrate Nernst-Planck Equation more easily
- Steady-State Condition: the net ionic current across the membrane is 0 (the membrane potential is stable over time)
step1 Get the Ji equation about Cioutsideand Ciinside
From Nernst-Planck Equation:
Ji=−Di(dxdCi+RTziFCidxdV)
- Ji: Flux of ion i (mol⋅m−2⋅s−1)
- Di: Diffusion coefficient of ion i in the membrane
- x: Position
- zi: Valence of ion i
- F: 法拉第常数
- R: 气体常数
- T: 绝对温度(K)
- V: potential
Apply the Constant Field Assumption:
Because of the assumption: dxdV=−dVm where Vm=Voutside−Vinside
so, substitute dxdV into the Nernst-Planck Equation:
define α=RTdziFVm
Ji=−Di(dxdCi−αCi)
here, we time e−αx on both sides:
∵e−αxJi=−Di(dxdCie−αx−αeαxCi)∴−Die−αxJi=dxdCie−αx−αe−αxCi∴−Die−αxJi=∂x∂(Cie−αx)
Now, we can integrate both sides from x=0(inside) to x=d(outside)
−DiαJi∫x=0x=de−αxdx=(Cie−αx)∣x=0x=d∴DiαJi(e−αd−1)=Cioe−αd−Cii∴Ji=−eαd−1Diα(Cio−Ciieαd)
We use Cio and Cii to represent the concentration of the given ion inside and outside the membrane.
Then recall that α=RTdziFVm, and substitute dDi=Pi (represent the permeability of ion i)
Ji=−RT(eRTziFVm−1)PiziFVm(Cio−CiieRTziFVm)
We use ξi=RTziFVm, so we can get:
Ji=−Piξieξi−1Cio−Ciieξi
Step2 get Vm
The ionic current Ii per square is
Ii=ziFJi∴Ii=−RT(eξi−1)Pizi2F2Vm(Cio−Ciieξi)
Apply Ii to the Steady-State Condition, where
∑Ii=Itotal=0
we use K+, Na+ and Cl− as examples, which are significant for the membrane potential. Here it is:(let ξ=RTFVm)
IK+=−RT(eξ−1)PK+F2Vm(CK+o−CK+ieξ)INa+=−RT(eξ−1)PNa+F2Vm(CNa+o−CNa+ieξ)ICl−=−RT(e−ξ−1)PCl−F2Vm(CCl−o−CCl−ie−ξ)=−RT(eξ−1)PCl−F2Vm(CCl−i−CCl−oeξ)
So the sum of the currents is
∑Ii=IK++INa++ICl−=0
substitute the currents:
−RT(eξ−1)PK+F2Vm(CK+o−CK+ieξ)−RT(eξ−1)PNa+F2Vm(CNa+o−CNa+ieξ)−RT(eξ−1)PCl−F2Vm(CCl−i−CCl−oeξ)=0⇒PK+CK+o+PNa+CNa+o+PCl−CCl−i=eξ(PK+CK+i+PNa+CNa+i+PCl−CCl−o)∴eξ=PK+CK+i+PNa+CNa+i+PCl−CCl−oPK+CK+o+PNa+CNa+o+PCl−CCl−i
recover ξ with RTFVm
Vm=FRTlnPK+CK+i+PNa+CNa+i+PCl−CCl−oPK+CK+o+PNa+CNa+o+PCl−CCl−i
Vm=ECl=−65mV
通透性与离子通道开放的个数有关,在RMP与AP下,通透性不同,所以膜电位也会相应改变
P*K:P_na:Pcl
At rest PK:P∗Na:P∗cl=1:0.04:0.45
Action potential PK:P∗Na:P_cl=1:20:0.45